Unleashing the Potential of γδ T Cells Through Checkpoint Blockade
In a groundbreaking study published in Nature Cancer, Hans R. Widlund and Lydia Lynch reveal that γδ T cells, a type of immune cell that has previously been overlooked, play a crucial role in the effectiveness of cancer immunotherapy. The researchers found that a specific subset of these cells, Vδ1+, is associated with better responses to immune checkpoint blockade (ICB) therapy in melanoma tumors with a lower neoantigen load.
ICB therapy has been a game-changer for patients with certain types of advanced-stage cancers, but predicting which patients will benefit most from these treatments remains a significant challenge. Conventional wisdom suggests that ICB therapy is beneficial in cancers with a high tumor mutational burden, such as melanoma, non–small-cell lung cancer, and gastric carcinoma. However, Widlund and Lynch's study challenges this notion by demonstrating that Vδ1+ γδ T cells are associated with improved responses to ICB in tumors with a lower neoantigen burden.
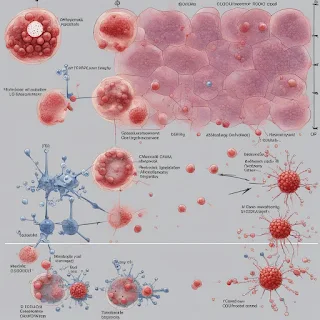
γδ T cells are a type of T cell that does not typically recognize antigens presented by major histocompatibility complex (MHC) molecules. Instead, they recognize stress ligands on tumor cells via their NKG2D receptors and other natural killer-like receptors. The study found that ligand recognition by NKG2D may be sufficient to upregulate PD-1 on Vδ1+ cells, and engagement of PD-1 by PD-L1 may hinder some of their anti-tumor functions. Intriguingly, ICB therapy can unleash their potent cytotoxicity, which would occur in the absence of MHC class I and neoantigens presented by MHC class I.
Furthermore, the study found that PD-1 expression on Vδ1+ T cells could be a result of chronic stimulation of the γδ TCR or activation of NKG2D. However, it remains unclear whether PD-1 is upregulated on Vδ1+ T cells after antigen recognition or through activation via the recognition of stress ligands by NKG2D. Therefore, further investigation is needed to determine whether Vδ1+ cells in the tumor become exhausted in the classic sense, with diminished cytolytic effector function.
In conclusion, Widlund and Lynch's study sheds new light on the role of γδ T cells in responses to ICB therapy. The researchers propose that Vδ1+ cells are particularly important in tumors with low neoantigen burden and in mismatch-repair-deficient tumors expressing mutant β2-microglobulin (β2M) that have exceedingly low antigen presentation by MHC class I and evade conventional surveillance by CD8+ T cells. These findings have significant implications for the development of future cancer immunotherapies.
Source: <https://www.nature.com/articles/s43018-024-00735-y>
ICB therapy has been a game-changer for patients with certain types of advanced-stage cancers, but predicting which patients will benefit most from these treatments remains a significant challenge. Conventional wisdom suggests that ICB therapy is beneficial in cancers with a high tumor mutational burden, such as melanoma, non–small-cell lung cancer, and gastric carcinoma. However, Widlund and Lynch's study challenges this notion by demonstrating that Vδ1+ γδ T cells are associated with improved responses to ICB in tumors with a lower neoantigen burden.
γδ T cells are a type of T cell that does not typically recognize antigens presented by major histocompatibility complex (MHC) molecules. Instead, they recognize stress ligands on tumor cells via their NKG2D receptors and other natural killer-like receptors. The study found that ligand recognition by NKG2D may be sufficient to upregulate PD-1 on Vδ1+ cells, and engagement of PD-1 by PD-L1 may hinder some of their anti-tumor functions. Intriguingly, ICB therapy can unleash their potent cytotoxicity, which would occur in the absence of MHC class I and neoantigens presented by MHC class I.
Furthermore, the study found that PD-1 expression on Vδ1+ T cells could be a result of chronic stimulation of the γδ TCR or activation of NKG2D. However, it remains unclear whether PD-1 is upregulated on Vδ1+ T cells after antigen recognition or through activation via the recognition of stress ligands by NKG2D. Therefore, further investigation is needed to determine whether Vδ1+ cells in the tumor become exhausted in the classic sense, with diminished cytolytic effector function.
In conclusion, Widlund and Lynch's study sheds new light on the role of γδ T cells in responses to ICB therapy. The researchers propose that Vδ1+ cells are particularly important in tumors with low neoantigen burden and in mismatch-repair-deficient tumors expressing mutant β2-microglobulin (β2M) that have exceedingly low antigen presentation by MHC class I and evade conventional surveillance by CD8+ T cells. These findings have significant implications for the development of future cancer immunotherapies.
Source: <https://www.nature.com/articles/s43018-024-00735-y>

Comments
Post a Comment